RSV vaccines likely approved by FDA this year: a successful story of structural vaccinology
Image from Unsplash
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection in infants and in elderly adults. Thus far, there is still no licensed vaccine for RSV. However, this is likely to change this year. In 2022, Pfizer’s bivalent RSV vaccine demonstrated 81.8% efficacy among infants in the first 90 days of life through vaccinating the mothers during pregnancy and 85.7% in elderly adults in the phase III vaccine trial. GSK also announced its vaccine achieved 82.6% efficacy among the older population. The U.S. FDA is expected to approve both vaccines for use in older adults by May and Pfizer’s vaccine use in pregnant women in August.
Each year during winter, we expect to see children's hospitals flooded with babies sick from the RSV infection and last year was a rough one with the RSV pandemic along with flu and COVID-19. RSV usually only causes mild upper respiratory tract infections in healthy adults, but it can cause life-threatening symptoms such as bronchitis and pneumonia in young children and elderly adults. About 90% of children will get infected by RSV at some point in the first 2 years of life, and about 1 to 2% of the infected babies younger than 6 months will require supportive care in a hospital. With oxygen and mechanical ventilation, RSV infection doesn’t usually cause death. Yet, in resource-limited countries that lack oxygen and ventilator, RSV is the second leading cause, following malaria, of infant death after the neonatal period — it claims around 101,400 lives of children younger than 5 years old each year.
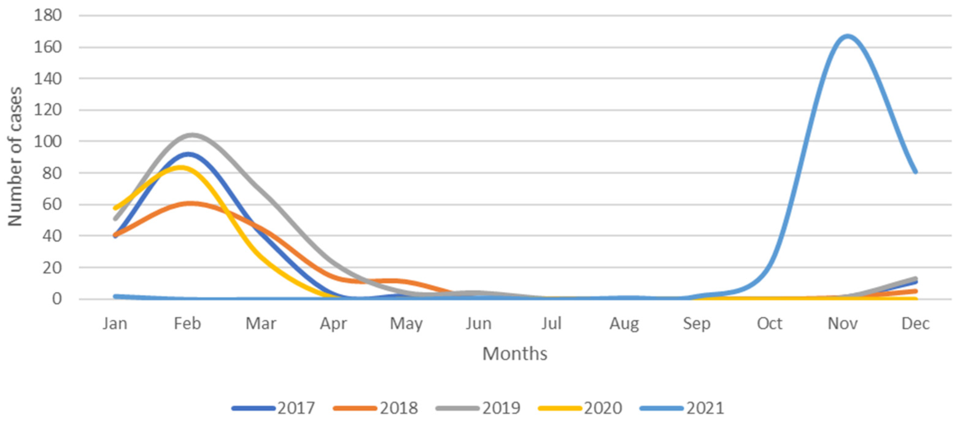
The number of children hospitalized for RSV-associated illness between 2017 and 2021 in the Policlinico-Giovanni XXIII Hospital of Bari in Southern Italy. Image from Children by Loconsole, Daniela et al.
Encouraged by several successful developments of inactivated vaccines, researchers in the 1960s tested the first formalin-inactivated RSV vaccine in infants. However, it failed miserably. The vaccine did not only fail to protect the infants but also exacerbated the illness caused by RSV. Among the infected, 16 were hospitalized and 2 of them died from RSV. Retrospective studies found that the antibodies induced by the formalin-inactivated vaccine did not neutralize the virus. Instead, by binding to the virus, these antibodies enhanced the illness. Such an effect, known as antibody-dependent enhancement (ADE), has also been observed in people who get infected for the second time by Dengue virus. The antibodies induced by the first Dengue infection enhance the illness during the second infection, and some of them may develop a severe form of Dengue infection — Dengue hemorrhagic fever.
Such catastrophic failure of the formalin-inactivated vaccine deterred vaccine scientists from developing new RSV vaccines until the 1990s and 2000s. Thanks to the advancement of subunit vaccines, scientists started to develop RSV vaccines based on RSV’s F protein, the essential protein for the virus to fuse with the target cells to initiate infection. Mark J. Kwakkenbos et al. in 2010 isolated a powerful antibody named D25 against both RSV strain strains of both A and B subtypes from an individual recovered from RSV infection. In 2013, by studying the structure of F protein bound by the D25 antibody, Jason S. McLellan and Barney S. Graham revealed that D25 can neutralize RSV by locking its F protein in the structure before it fuses with the target cell, a structure is now known as pre-fusion F protein. In the same year, McLellan and Graham designed a vaccine based on the pre-fusion F protein and proved that it can elicit antibodies that neutralize RSV in mice and monkeys after immunization. The phase I trials with the results published in 2019 and 2021 demonstrated that the pre-fusion F protein vaccine is safe and can induce strong neutralizing antibodies against RSV.
From fatal failure to today’s success in the phase III vaccine trial, the development of RSV vaccines tells a successful story of structural vaccinology.
The structure of RSV’s pre-fusion F protein bound by the D25 antibody. Image from Science by Jason S. McLellan et al.